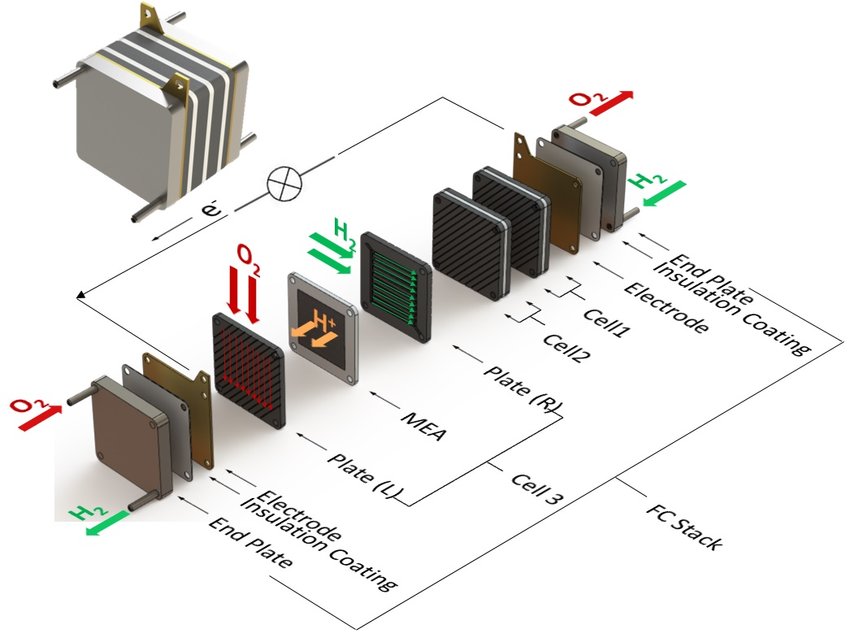
Hydrogen fuel cells have gained increasing attention as a sustainable energy source due to their high energy efficiency and low environmental impact. A fuel cell operates by converting the chemical energy from hydrogen fuel and oxygen into electricity, heat, and water. The key components of a fuel cell include the membrane electrode assembly (MEA), the fuel diffusion layer, the oxygen diffusion layer, the bipolar plate, and the stack.
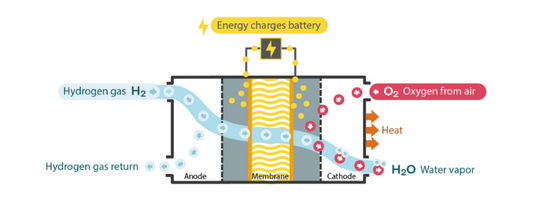
Carbon paper is commonly used as the gas diffusion layer in hydrogen fuel cells. The purpose of the gas diffusion layer is to allow the gases to diffuse uniformly over the surface of the electrode while also acting as an electrical conductor. Carbon paper is a good material for this purpose because it has a high porosity, high electrical conductivity, and is chemically inert. However, the high cost of carbon paper limits its widespread use in fuel cells.
Bipolar plates are used to connect multiple cells in a fuel cell stack and to distribute reactants and products. Bipolar plates must be electrically conductive, corrosion-resistant, and have a low electrical resistance. Several materials have been evaluated for use as bipolar plates, including graphite, metals, and composites. Composite bipolar plates made from a mixture of graphite and thermoplastic polymers offer a good balance of mechanical strength, electrical conductivity, and corrosion resistance.
In summary, hydrogen fuel cells offer a promising energy source for the future, with carbon paper playing a key role as the gas diffusion layer and composite bipolar plates offering a cost-effective solution for connecting multiple cells in a fuel cell stack. Ongoing research is focused on developing more efficient and durable fuel cells with improved materials and designs.